Aseptic production of milk and juice
The intention of this book is to present some of the knowledgeacquired during many years of practical work on the qualitycontrol and quality assurance of long-life products. Differentfactors have an impact on product quality – the standard of the rawmaterials, the equipment involved, the processes applied, opera-tional procedures, installation, and so on. In order to produce ahigh quality product, a thorough understanding of these factors isnecessary. An attempt has been made to discuss and present thematerial in a comprehensible way concentrating on microbio-logical aspects. The HACCP (QACP) concept has been used as amodel for process control.UHT-treated and aseptically packaged products have beenproduced in large quantities for quite some time. So far, the litera-ture available concentrates on particular aspects of product andequipment characteristics and the technology involved. A moregeneral presentation appears to be lacking. Hopefully, our workwill help close the gap.To a large extent, the available “old” literature has beenconsulted. The new literature is readily accessible through dataprofile searches. In addition, more than 25 years of practical fieldexperience has gone into this study.The different topics are arranged in such a way that each chap-ter stands as an independent entity. Of necessity, this has led tosome repetitions. We hope that the reader will accept the problemand make allowances for the consequences. It is also our hope thatthis study will contribute to the production of long-life productswith a high level of quality.Finally, we would like to express our appreciation to all theTetra Pak people who have helped to prepare this book and whohave contributed with valuable comments on the contents. Thisapplies particularly to Gösta Odelberg B. Sc., Regional ManagerFiSQA (Field Service Quality Assurance) and Dr. Charles Sizer.Åkarp, August 1998
Dr. Bernhard von Bockelmann Dr. Irene von Bockelmann
You can use cash or points to buy this book at points store or related display area. English version and name is Quality management of aseptic production, Chinese version and name is 无菌生产质量管理.
00.Introduction
Commercially sterile products have been on the market for a long time. Direct
UHT (Ultra High Temperature) processing was introduced at the beginning ofthe 20th century. At that time, the technology was used only as a pre-treatmentfor products to be filled into cans for subsequent retorting. The first UHT-treatedand aseptically packaged products were in cans and were shown at an agricultural exhibition in London in the mid 1920s. The product (milk) was not a commercial success. During the early 1930s, aseptic canning was developed in the USA (69).
In 1961, aseptic packaging procedures were introduced (74, 93, 243) using flexible packaging material (a laminate of wax, paper and polyethylene): theTetra Pak system (“Tetra Classic Aseptic” or TCA system). The paperboardcarton and black colouring provided protection against the entry of light. How-ever, gas barrier characteristics were rather poor. In the meantime, UHT process-ing systems were in more general use. After initial problems, the combination ofUHT processing and aseptic packaging in the TCA system gained market success(“long-life products”).
In 1969, a brick-shaped package was introduced (“Tetra Brik Aseptic” or TBAsystem). The structure of the packaging material was more complex: a poly-ethylene-paper-polyethylene-aluminium foil-polyethylene laminate. Such amaterial provides protection against the entry of light and acts as an effective gasbarrier. The shape of the container offered considerable advantages in storage,transportation and handling. It also provided the real break through for UHTprocessing and aseptic packaging technology. Today, larger quantities of both low and high-acid, shelf-stable food products are processed by inflow (UHT)methods and subsequently packaged under aseptic conditions. Milk andmilk-based products were the first long-life food products and still remain thelargest commodity in terms of volume.
Processes that stabilise food thermally have been a major benefit to mankindby providing stability and safety in food. Although commercial canning isprobably the most reliable and safest method of food preservation, it is not perfect. In trying to improve the technology, the present low risk of contamina-tion present in simple straight forward technologies must sometimes be sacrificed to gain better quality and economy.
Aseptic technology is considered to have the followingadvantages over conventional technologies (254):
a)new package forms;
b)savings in energy and packaging costs;
c)convenience; and
d)improved product quality.
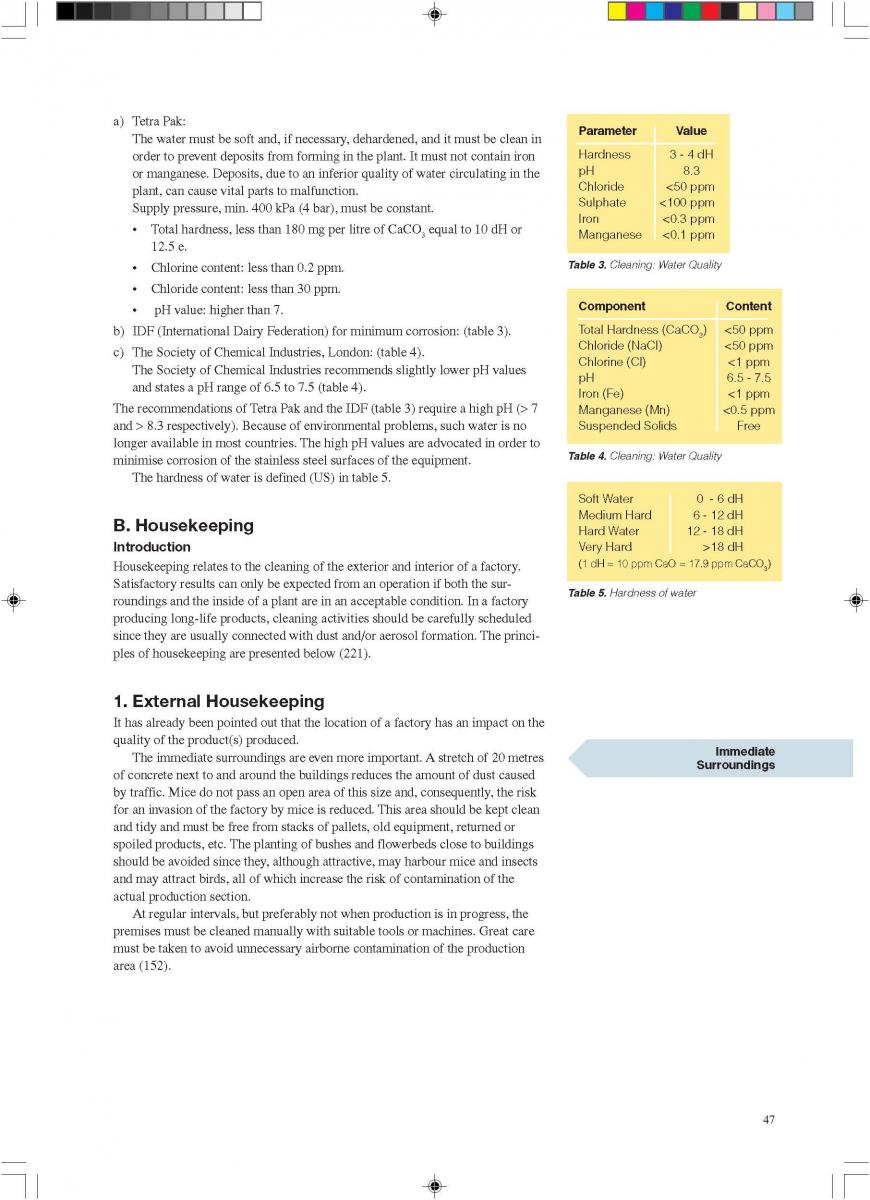
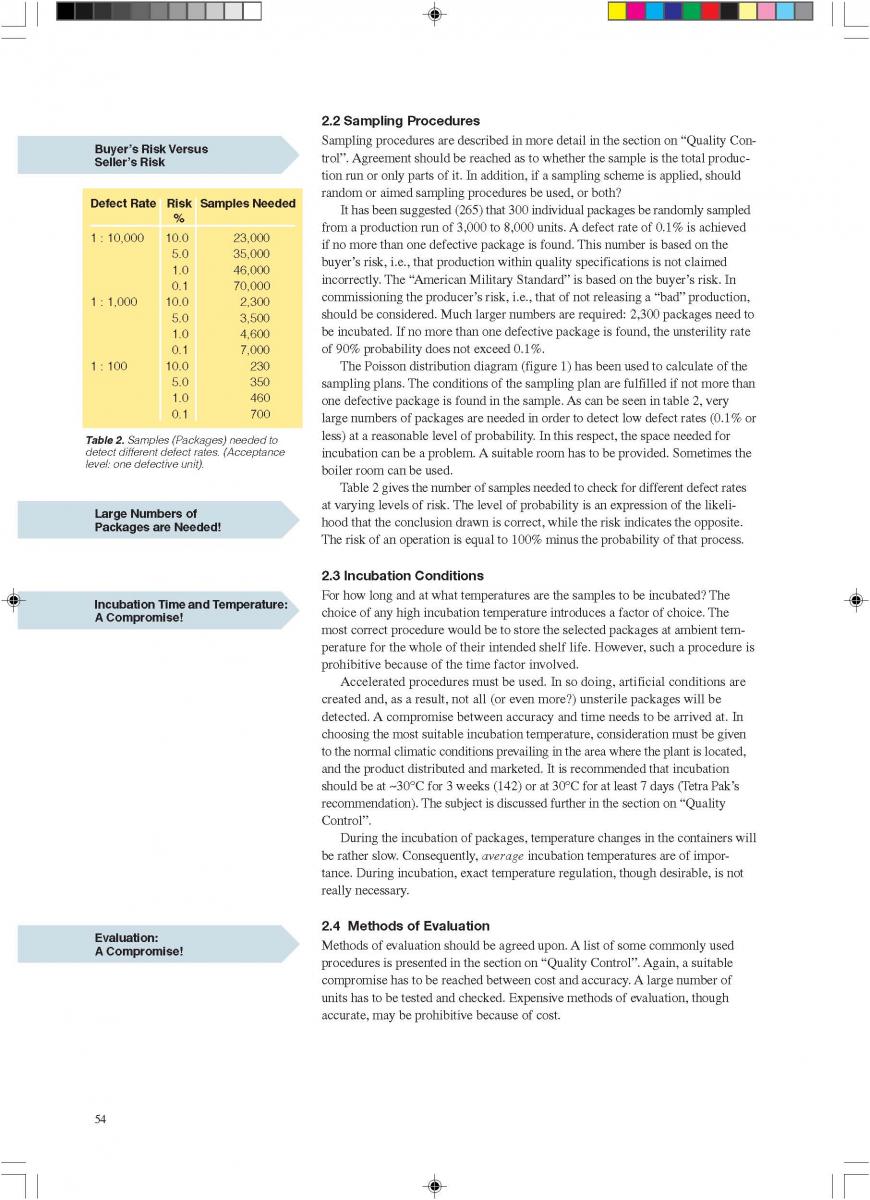
A comparison of the price of different packaging alternativesis shown in table 1 (278).In table 2, the total cost of canning is compared to theproduction of a long-life food product (table 2, 179).
01.UHT processing
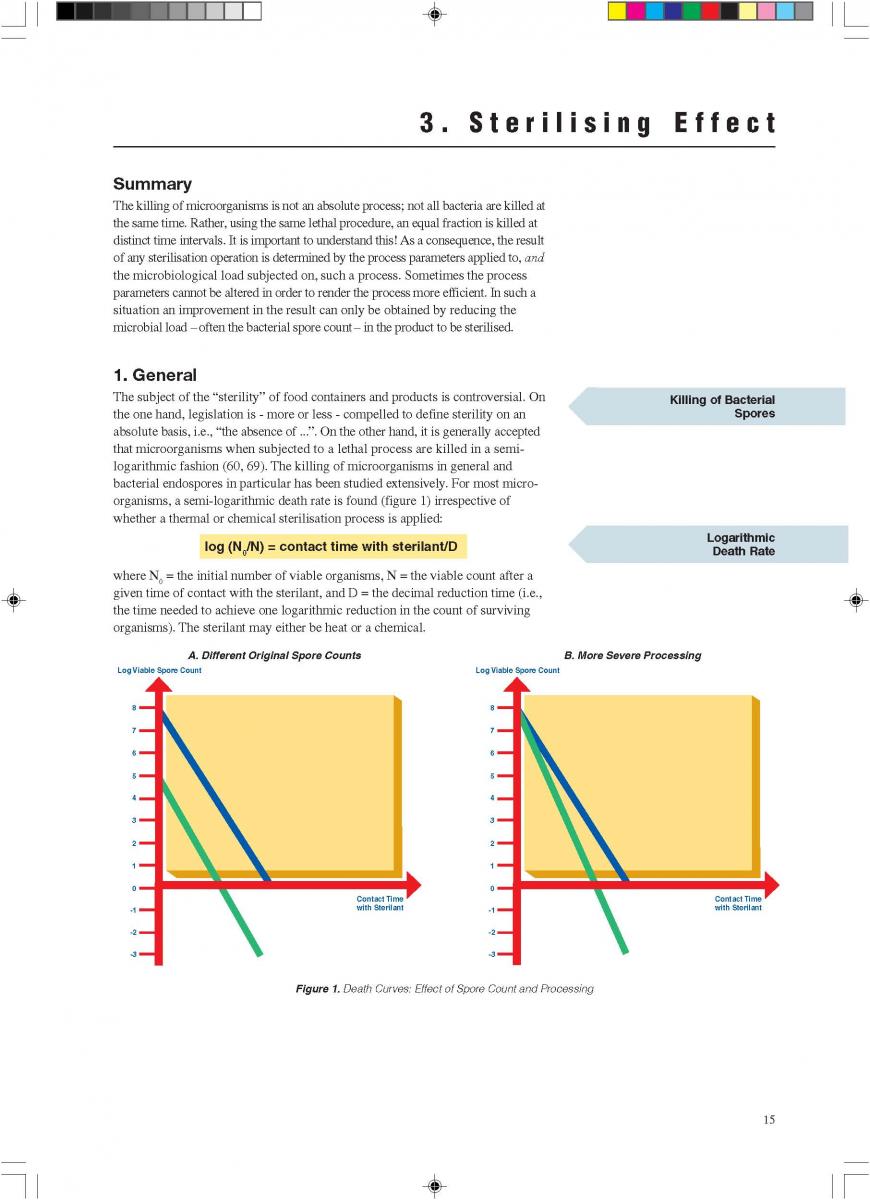
Summary
Food products are processed by UTH Methods in order to obtain a commercially sterile product. Rapid heating, short holding and rapid cooling minimise the occurrence of chemical change. A short description is given of the principles involved in indirect and direct heating systems.
011.General description
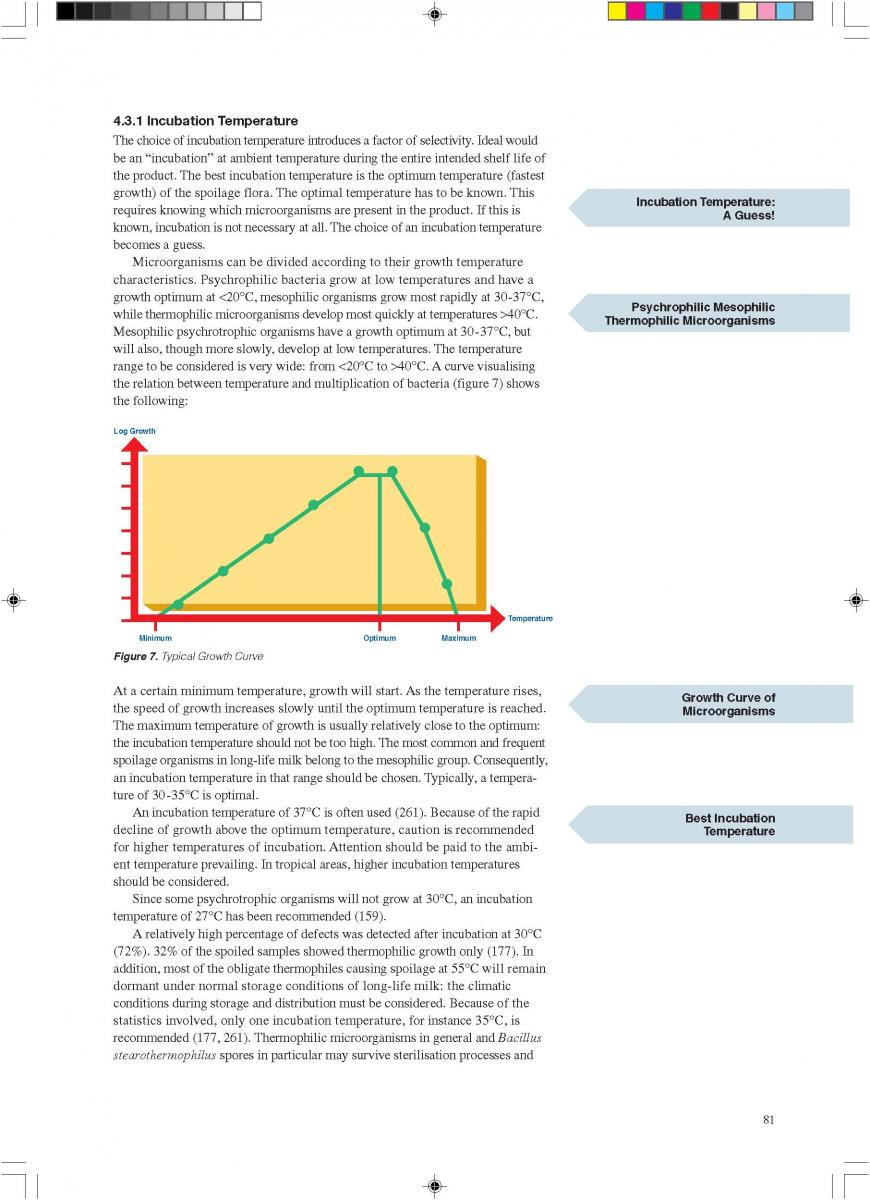
1. General
Ultra-high-temperature treatment is a continuous inflow
process. It is based on the rapid heating of the product to the sterilisation temperature and short holding at that temperature, followed by rapid cooling. The purpose of UHT treatment is to achieve commercial sterility of the product. Full sterilisation efficiency requires rapid heat transfer which is only possible in liquid systems. If powders are used in the formulation of a product to be UHT-treated, special attention has to be paid to proper soaking: all powder particles must be completely wetted through.
1.1 Low-Acid, Liquid-Food Products
Low-acid food products are characterised by a pH value of > 4.5 or > 4.6, depending on local food legislation. These products require careful treatment because:
a) microorganisms can grow and multiply. Bacterial spores are also able to germinate and cause product spoilage;
b) practically all pathogenic (disease-causing) microorganisms can develop.
Consumption of products thus spoiled may lead to food poisoning and/or food-borne infections.
Typical processing temperatures for low-acid foods are 130-150°C with holding times of a few seconds (60), usually 4 seconds.
1.2 High-Acid Liquid-Food Products
High-acid food products have a pH value equal to 4.5 or 4.6 or less. These are mainly fruit juices, fruit juice drinks and “belly washers”. High-acid food products are safer than low-acid foods because:
a) they are not prone to pathogenic bacteria and are therefore regarded as safe from the point of view of public health;
b) bacterial spores cannot germinate under high-acid conditions and, consequently, cannot cause food spoilage;
c) the sterilising efficiency of any heat treatment increases with decreasing pH. Thus, lower temperatures can be applied in order to achieve commercial sterility;
d) in addition, some organic acids common in fruits specifically decrease the temperature resistance of possible spoilage organisms;
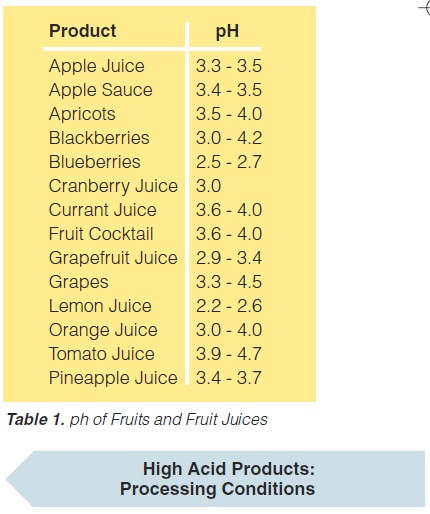
e) the main spoilage microorganisms are yeast, mould, and a few bacteria (Lactobacillus, Streptococcus, and some others). Processing temperatures for high-acid foods are rather low. With few exceptions temperatures of 85 -95°C with holding times of 30 to 15 seconds, sometimes a few minutes, are sufficient (60). However, there are some exceptions, particularly tomato products which may require considerably higher temperatures, often above 100°C. The pH values of a number of fruits and fruit juices (131) are given in table 1.
1.3 Acidified Liquid-Food Products
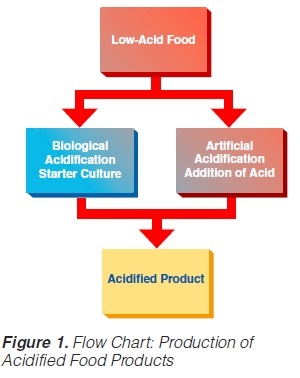
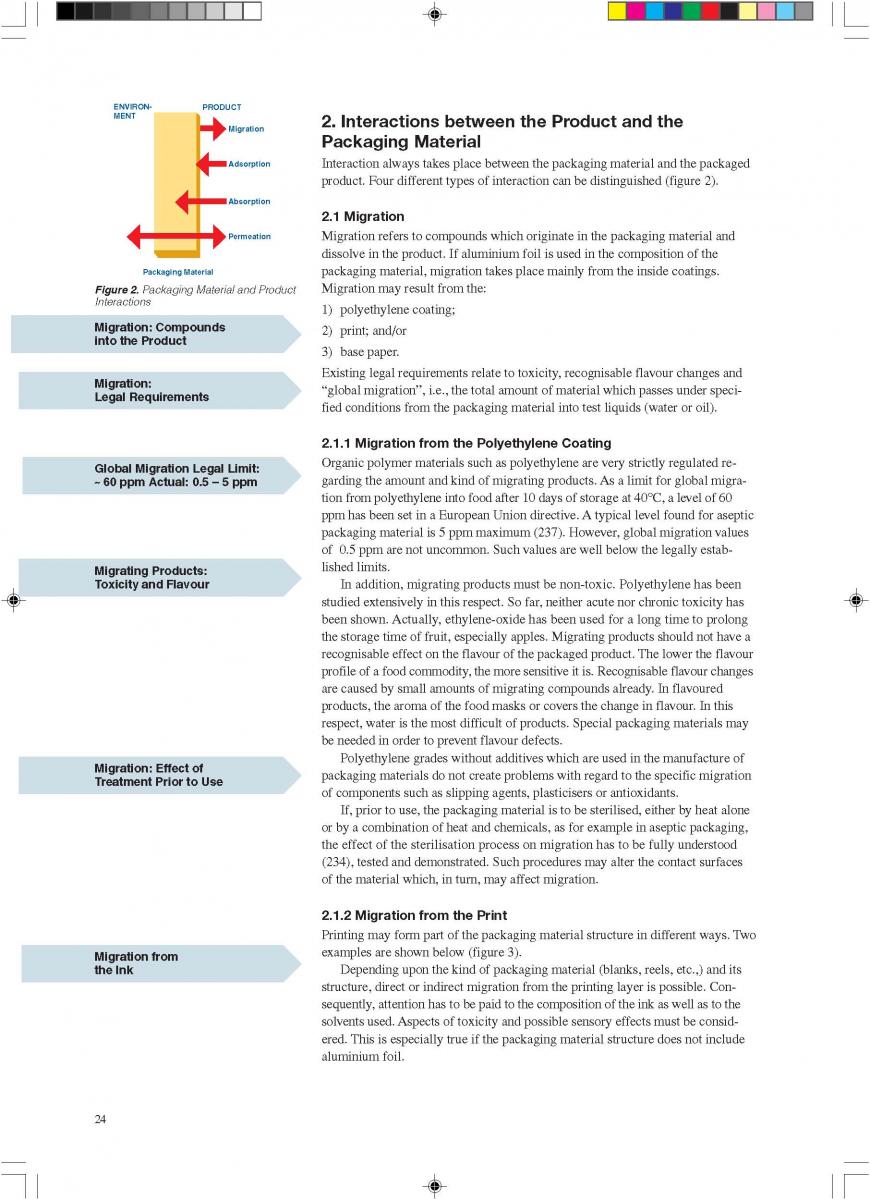
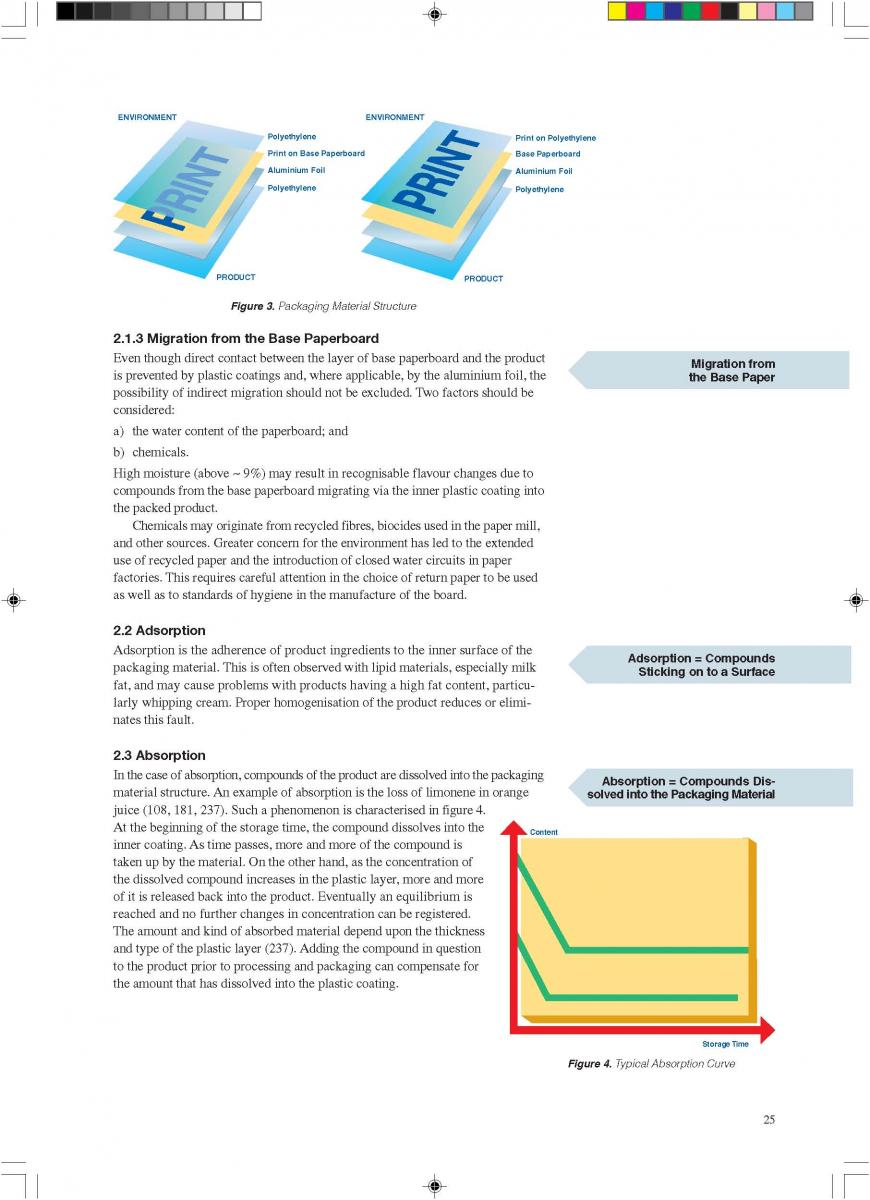
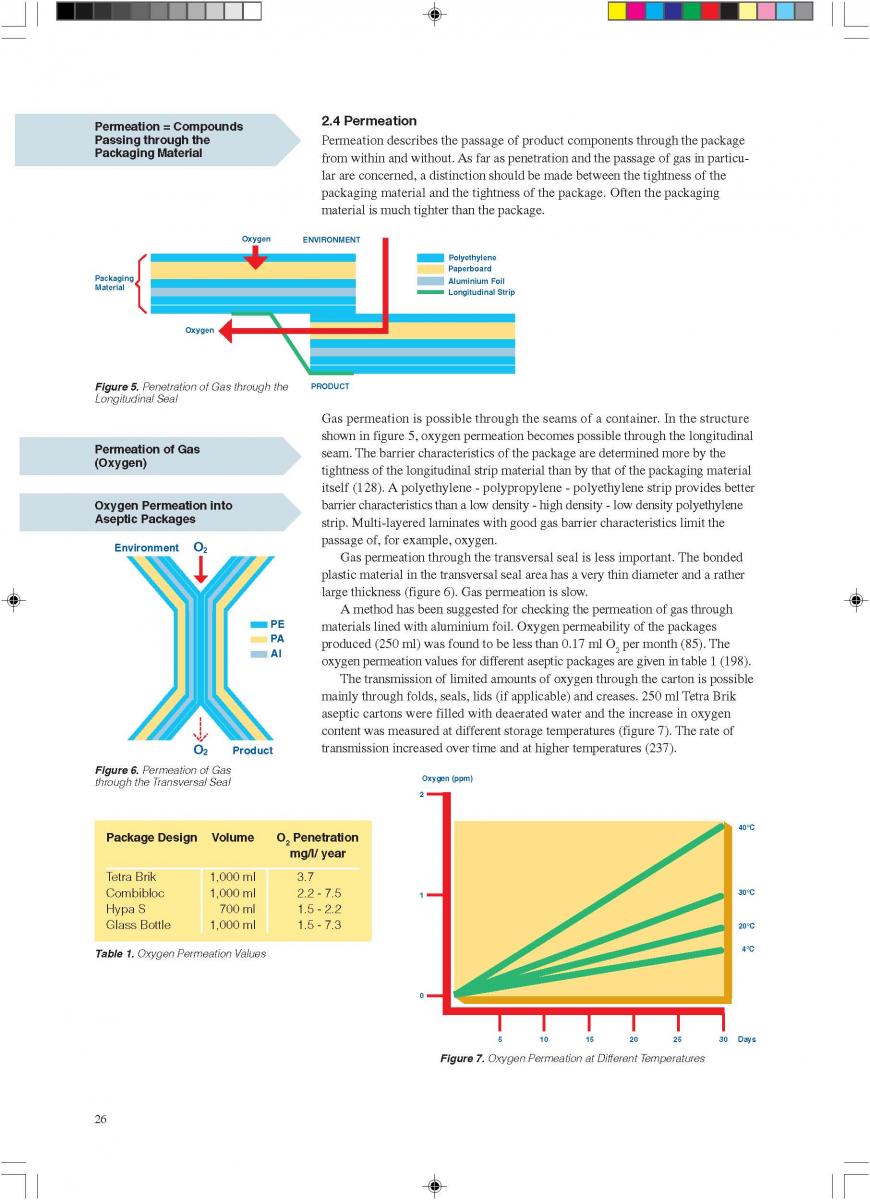
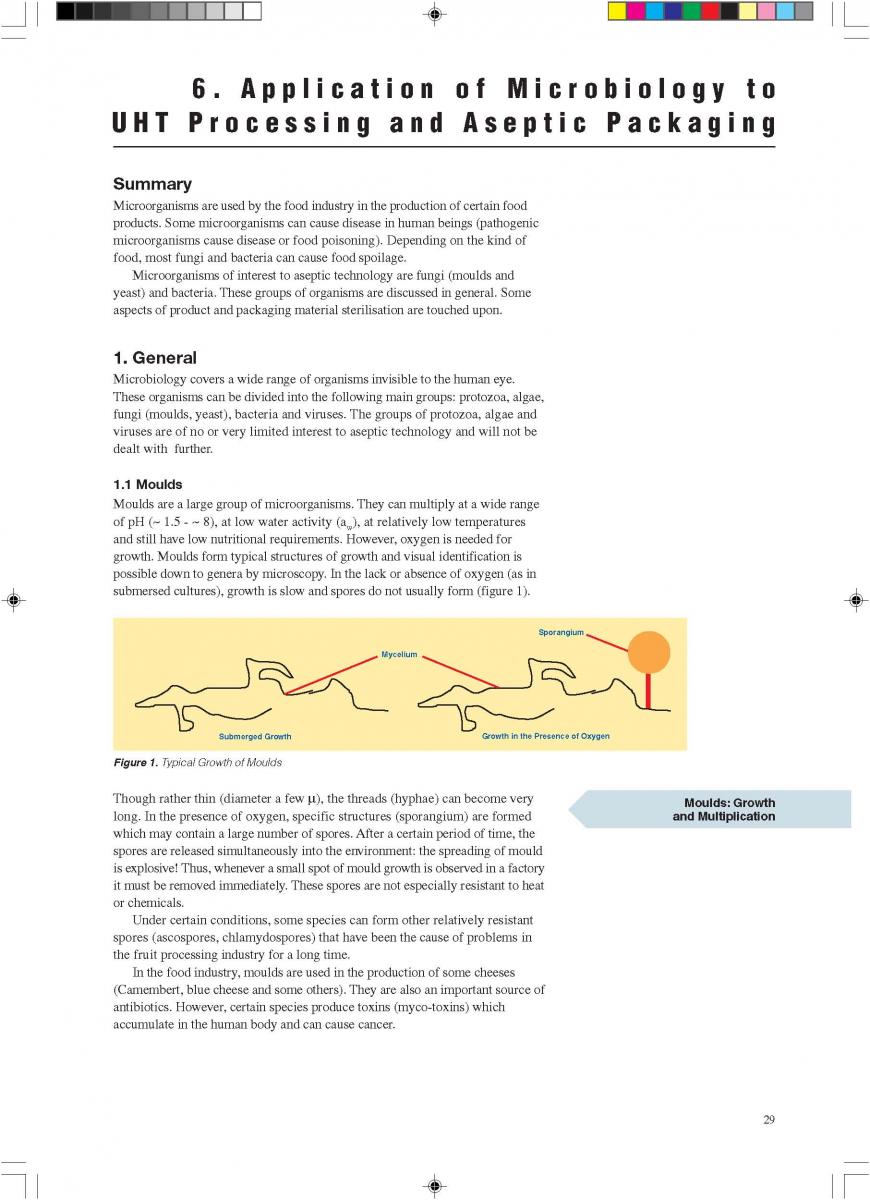
Acidified foods are low-acid products, the pH of which has been lowered into the high-acid range by pre-processing. Such products have the same microbiological advantages as high-acid food products. The acidification process is crucial. It is necessary to achieve an even and low pH throughout the product. Acidification can be carried out biologically by the addition and growth of a starter culture, usually Lactobacillus and/or Streptococcus followed by a ripening period at a suitable temperature. Another possibility is an artificial or chemical procedure in which an acid (usually citric or lactic acid) is added to the product which has to be mixed thoroughly afterwards (Figure 1). To achieve commercial sterility, the acidified product has to be processed after the acidification process, usually by heat treatment, and then packaged.
012.Indirect UHT
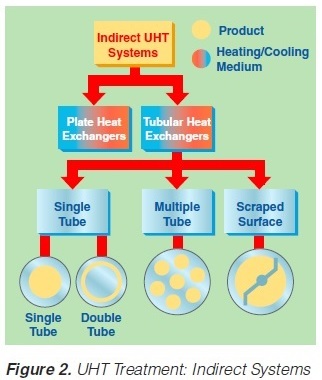 2. Indirect UHT Systems
2. Indirect UHT Systems
A heat exchange surface separates the product from the heating or cooling media in indirect UHT systems. The heating medium may be either steam or superheated water (Figure 2). A time-temperature diagram for indirect systems is shown in
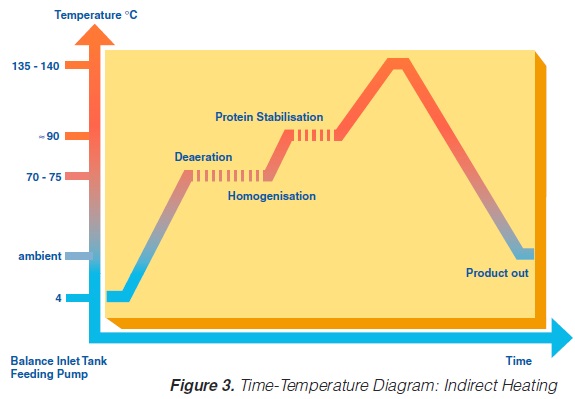
figure 3. Typically, the product enters the steriliser via a balance inlet tank and a centrifugal feeding pump at about 4°C. Subsequently, it is heated to 70 -75°C, at which temperature the product is homogenised. For milk homogenisation, pressures of 200 to 250 kg/cm2 (150 to 200 at the first stage, and about 50 kg/cm2 at the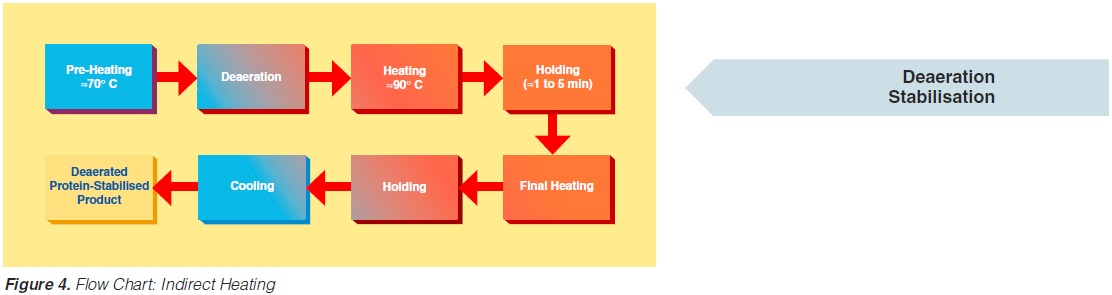 second) are often used. The omogeniser is a positive piston pump which pushes the product through the remaining equipment directly to the aseptic filling machine or into an aseptic tank for subsequent filling. Sterilisation temperatures are usually between 135°C and 140°C. Holding times between 2 and 6 seconds at the sterilisation temperature are common. Though more expensive, downstream (aseptic) homogenisation results in better flavour characteristics for some products. In order to lower the oxygen content of the product, deaeration can be introduced prior to upstream homogenisation. Milk entering the steriliser is normally saturated with oxygen. Since indirect working sterilisers are closed systems, the incoming and outgoing oxygen content of the product is the same. Depending upon temperature, milk may contain 6 to 9 ppm of oxygen (ca 7ppm), (168). Passing a vacuum chamber (deaerator) at high temperature prior to homogenisation (~ 70°C), the oxygen
second) are often used. The omogeniser is a positive piston pump which pushes the product through the remaining equipment directly to the aseptic filling machine or into an aseptic tank for subsequent filling. Sterilisation temperatures are usually between 135°C and 140°C. Holding times between 2 and 6 seconds at the sterilisation temperature are common. Though more expensive, downstream (aseptic) homogenisation results in better flavour characteristics for some products. In order to lower the oxygen content of the product, deaeration can be introduced prior to upstream homogenisation. Milk entering the steriliser is normally saturated with oxygen. Since indirect working sterilisers are closed systems, the incoming and outgoing oxygen content of the product is the same. Depending upon temperature, milk may contain 6 to 9 ppm of oxygen (ca 7ppm), (168). Passing a vacuum chamber (deaerator) at high temperature prior to homogenisation (~ 70°C), the oxygen
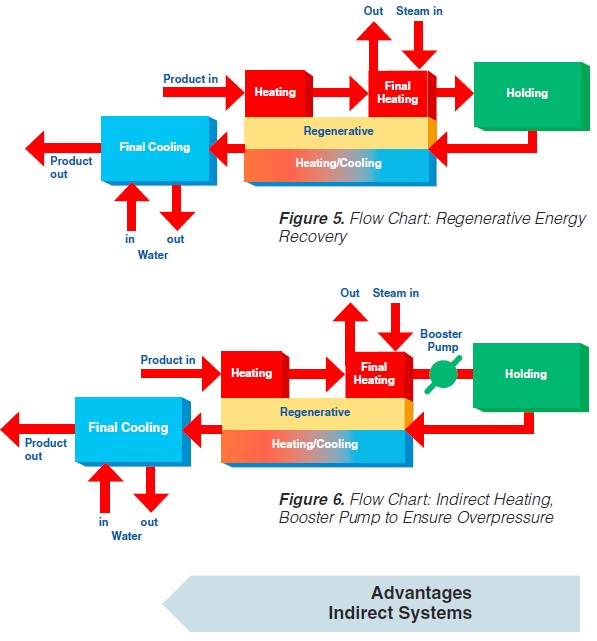
content can be reduced to about < 1 to 3 ppm (0.3 - 0.9 ppm), (168). The degree of deaeration depends upon the temperature and the underpressure applied in the process. During heat processing, deposits form on heat exchange surfaces, particularly in the temperature range > 80°C. In order to reduce this deposit formation and thus prolong the running time, a holding cell can be introduced: the product (milk) is held for a few minutes at a temperature of about 90°C (Figure 4). Indirect systems offer good possibilities for regenerative energy recovery: the incoming productis heated by the outgoing.
013.Direct UHT systems
Direct UHT Systems
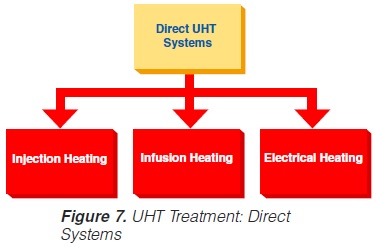 Direct UHT plants feature direct contact between the heating medium and the product. The heating medium is usually steam but electrical heating (“Elecster”, “Ohmic”) has been introduced to a very limited extent(figure 7).
Direct UHT plants feature direct contact between the heating medium and the product. The heating medium is usually steam but electrical heating (“Elecster”, “Ohmic”) has been introduced to a very limited extent(figure 7).
Electrical heating features the passage of an electrical current through the product. Although it is also used for liquids, the system is mainly intended to be applied in the sterilisation of products containing particles. Commercial application is very limited although a number of plants have been installed in the research and development departments of some major food and animal-feed processing companies. One problem is the difference in electrical resistance which can exist between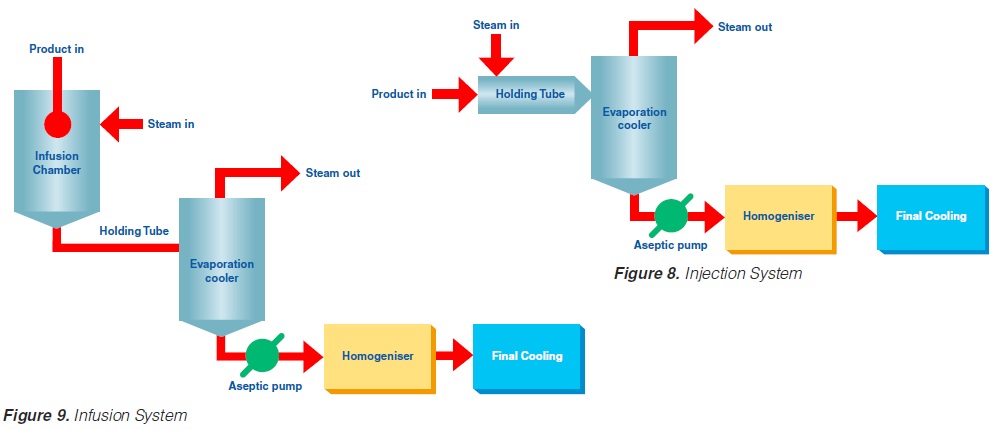 the liquid and solid phases. Other problems generally associated with the processing of products containing particles are those of separation during processing and aseptic transfer, and mechanical damage to particles softened by processing.
the liquid and solid phases. Other problems generally associated with the processing of products containing particles are those of separation during processing and aseptic transfer, and mechanical damage to particles softened by processing.
 Injection implies direct steam injection into the product. In infusion plants, the product is infused into a steam chamber.
Injection implies direct steam injection into the product. In infusion plants, the product is infused into a steam chamber.
 Injection and infusion systems must be operated with culinary steam.
Injection and infusion systems must be operated with culinary steam.
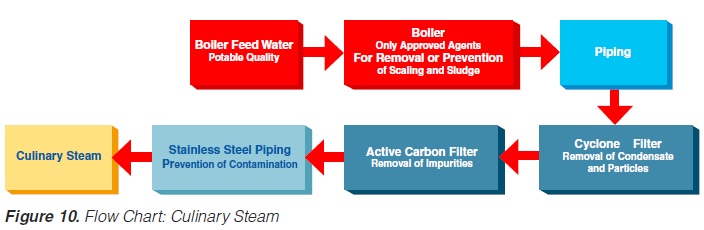
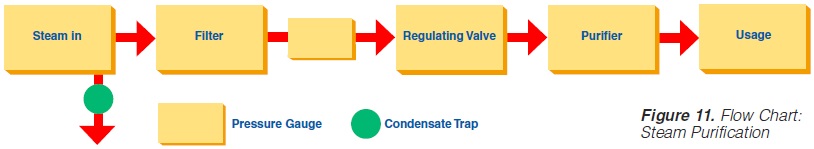
The minimum requirements outlined in figure 10 must be observed for the steam in such plants (not necessary for electrical heating). Indirect working plants should also use culinary steam especially if they are sterilised with steam.
The US Department of Health and Human Services (17) lists the following requirements for culinary steam:
1. Boiler Feed Water: If boiler feed water is treated, the process must be under the supervision of trained personnel. Only compounds complying with Section 173.310 of 21 CFR (258) may be used to prevent corrosion and scale.
2. Boiler Operation: A supply of clean, dry saturated steam is necessary for proper equipment operation. It is recommended that periodic analysis be made of condensate samples.
3. Piping Assemblies: The steam supply line should be as shown in figure 11.
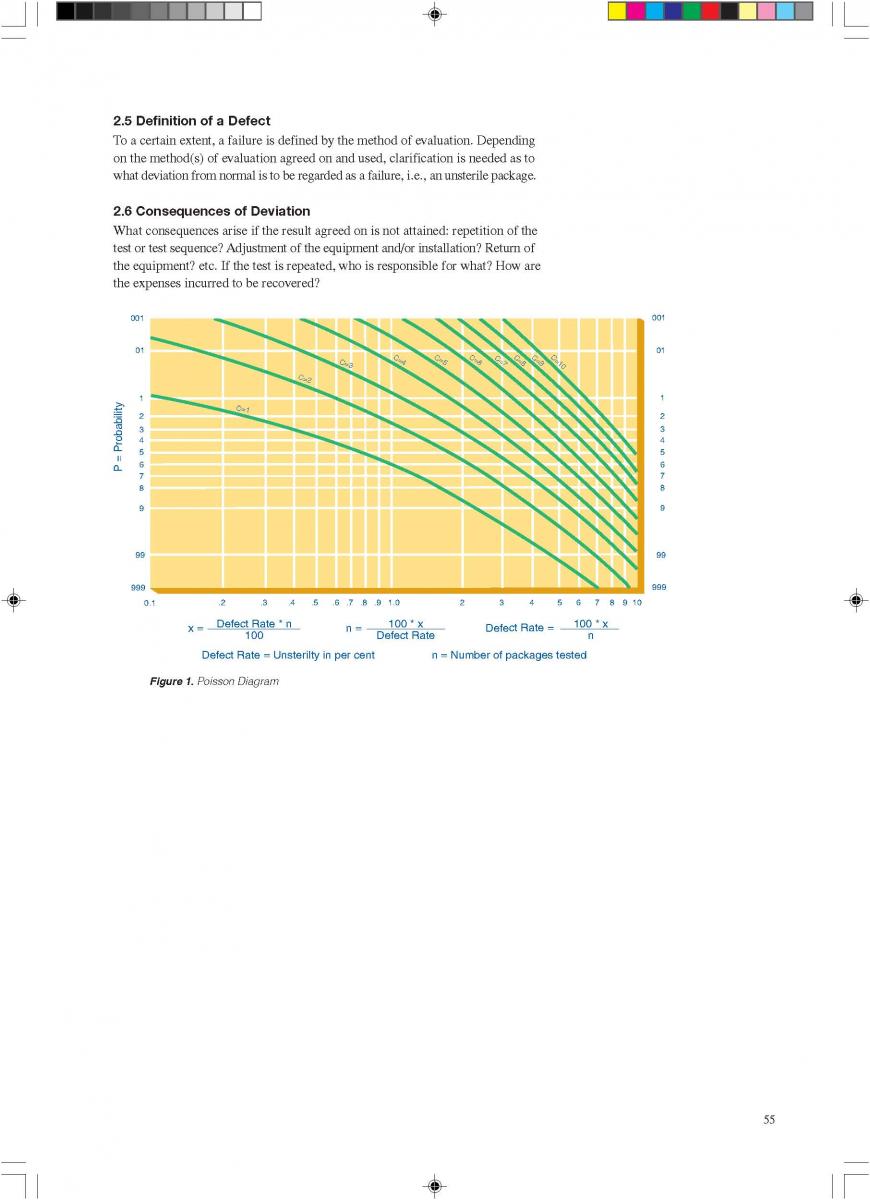
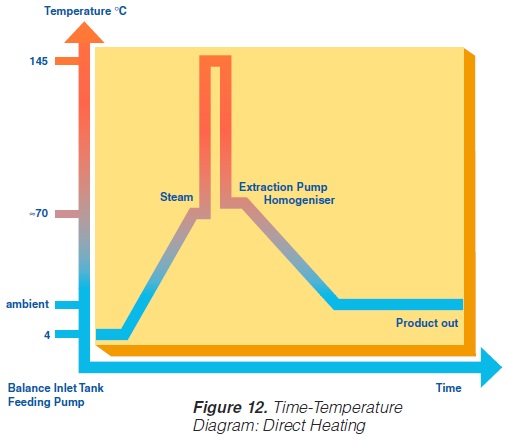 A typical time-temperature diagram for direct heating is shown in figure 12.
A typical time-temperature diagram for direct heating is shown in figure 12.
The product enters the steriliser via a balance inlet tank and a centrifugal feeding pump at a temperature of about 4°C. It is heated by plate or tubular heat exchangers to about 70°C. At this stage, steam is injected into the product or the product is infused into a steam chamber. Steam condensation increases the temperature almost instantaneously (~ 0.1 sec in injection and ~ 0.25 sec in infusion systems) to the sterilisation temperature which is typically between 145°C and 150°C. The average holding time at the sterilisation temperature is usually around 4 seconds. In both the injection and infusion processes, water condenses in the product and dilutes it. Depending on the temperature difference, an increase in volume of about 10% results. This water must be subsequently removed. The outlet of the holding cell connects to a vacuum chamber. To prevent boiling in the product holding cell, a sufficient overpressure by a suitable restriction device must be introduced. Being exposed to underpressure, the product starts boiling vigorously and steam is flushed off. Careful adjustment of the injection (infusion) temperature and the underpressure in the vacuum chamber guarantees the same dry matter content of the incoming and outgoing product. The resulting pressure drop requires the installation of an aseptic extraction pump for further product transportation. In order to avoid an accumulation of product in the expansion cooler or its emptying, the capacity of both the product feeding pump and the extraction pump at the outlet of the expansion cooler must be carefully
matched.
Cavitation forces during steam condensation and the boiling in the expansioncooler destabilise milk protein and fat. To compensate this effect requires downstream homogenisation which has to be done under aseptic conditions. Homogenisation pressures for milk are usually 200 to 250 kg/cm2 (150 to 200
kg/cm2 at the first stage and about 50 kg/cm2 at the second). The homogeniser pushes the product through the final cooling section of the steriliser, either into a sterile tank or directly to the aseptic filling machine.
In the expansion cooler, not only water is removed from the product but also all other volatile compounds. In addition, the vacuum chamber functions as a very effective deaerator that removes oxygen and other dissolved gases, mainly carbon dioxide (CO2). As a consequence, the freezing point increases. At the outlet of the expansion cooler, the oxygen content of milk is down to ~ 0.1 ppm.
 Advantages of injection and infusion heating are:
Advantages of injection and infusion heating are:
a) a lower total heat load, as a result of which fewer chemical changes are inflicted on the product;
b) less scaling, particularly in the temperature range of 70°C and above resulting in longer production runs (less frequent cleaning and sterilisation);
c) the low oxygen content in the product increases the stability of some vitamins and, during storage, reduces flavour changes caused by oxidation;
d) more suitable for viscous products.
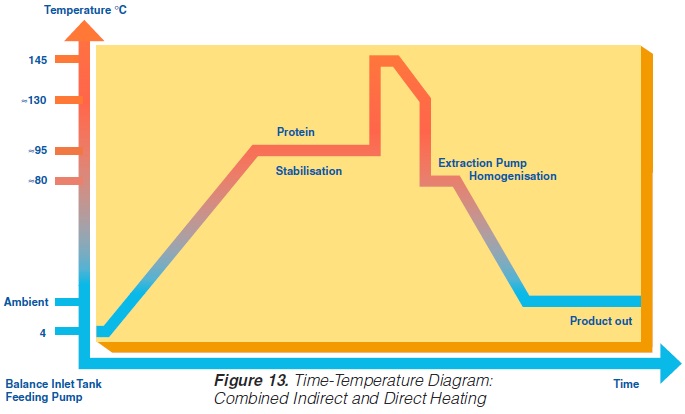
Recently, special UHT heat exchangers have been developed combining direct and indirect heating. In one assembly, tubular heating and steam injection have been combined. The product enters at ~4°C and is heated to 95°C by tubular heat exchangers. It then passes a holding cell at the same temperature to stabilise the protein. Steam injection raises the temperature almost instantly to 140-150°C. The product is held at this temperature for a few seconds before being cooled down. Pre-cooling is performed in a tubular heat exchanger where the heat energy is utilised for regenerative heating. The injected steam is flushed off as vapour in a vacuum cooler. The temperature drops to 80°C. Aseptic homogenisation is needed. Subsequently, the product is cooled down to ambient and filled aseptically.
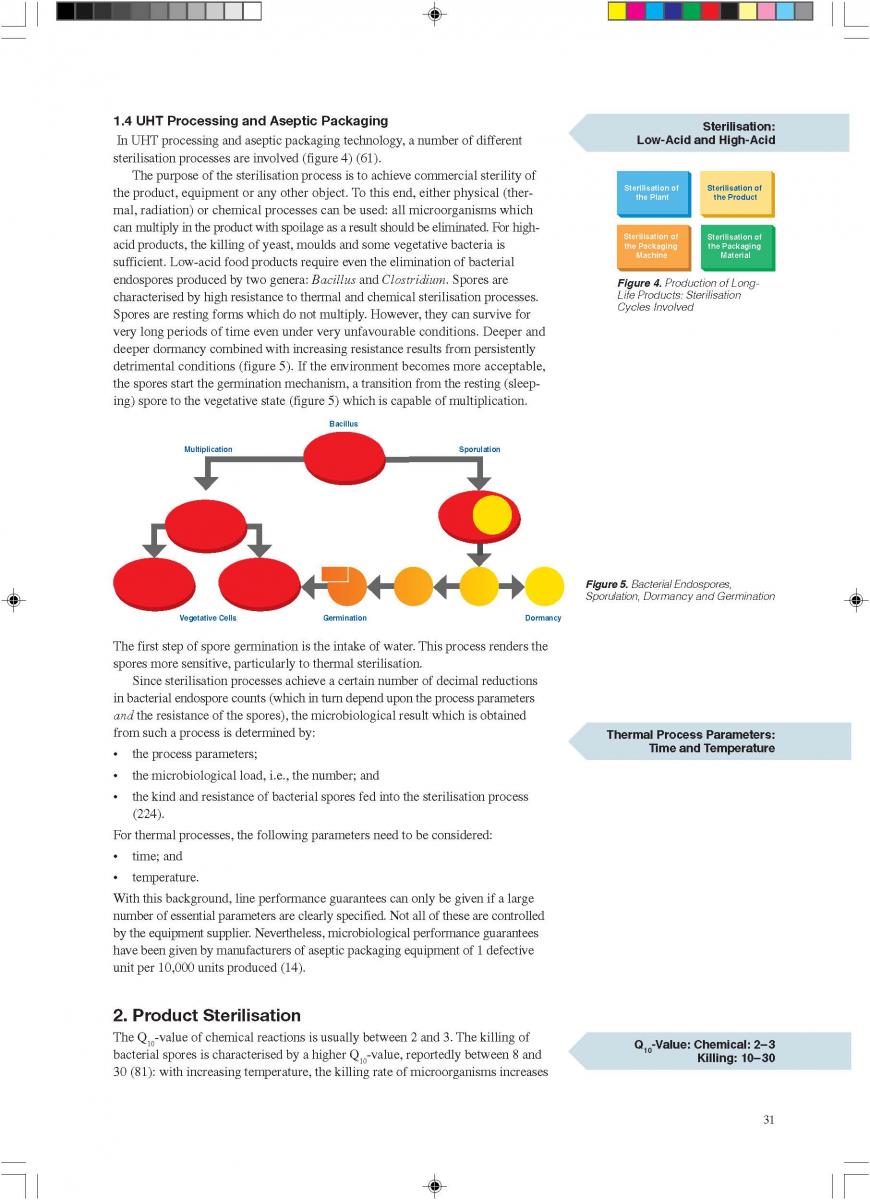
014. Plant Sterilisation
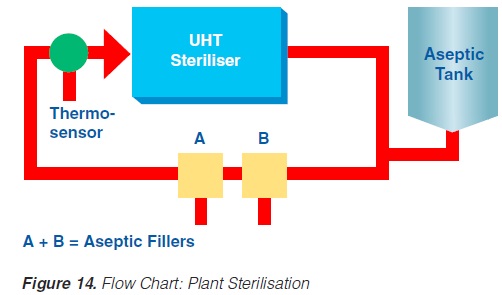 Prior to production, the equipment must be sterilised. This can be achieved either by superheated water or by steam. Because of the expansion and infusion vessels, direct working systems must be sterilised by steam. It is essential that the temperature controller/guarding sensor is placed at the most sensitive spot, usually somewhere in the return.
Prior to production, the equipment must be sterilised. This can be achieved either by superheated water or by steam. Because of the expansion and infusion vessels, direct working systems must be sterilised by steam. It is essential that the temperature controller/guarding sensor is placed at the most sensitive spot, usually somewhere in the return.
If superheated water is used to sterilise the plant, the temperature, time, and flow rates are critical (142). Possible air pockets and the interface between the product steriliser and the aseptic tank circuit are significant. Air is effectively removed from the system if the flow rate is > 1.5 m/second.
An additional sterilisation circuit is required in installations operating through an aseptic tank. The sterilisation medium is always steam. In steam sterilisation processes, a possible problem is condensate which must be removed from the system.
Sterilisation of the aseptic filling equipment is always performed separately either by heat alone or by chemicals and heat. Attention should be paid to the interface between the product line and the filler.
02.Aseptic packaging
Requires 90 POINTS in the General category.021. General
 UHT processing results in a commercially sterile product. The task of the aseptic packaging operation is to:
UHT processing results in a commercially sterile product. The task of the aseptic packaging operation is to:
a) maintain the high microbiological quality of the product for the length of its intended shelf life; and
b) retain consumer acceptance with regard to the flavour, texture and nutritional value of the product during its promised shelf life.
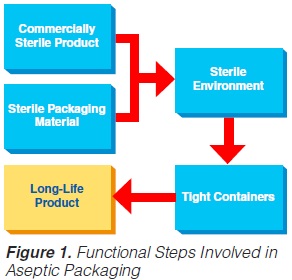
Four different principles of aseptic packaging are distinguished (74):
a) filling in a sterile working area;
b) aseptic filling into pre-formed containers;
c) aseptic filling into pre-formed sterile containers; and
d) the aseptic form-fill-and-seal method.
By and large, only the aseptic form-fill-and-seal method will be covered, which is also the best and most successful technology. Such an aseptic packaging operation requires several functional steps (figure 1) (60, 61, 74, 86).
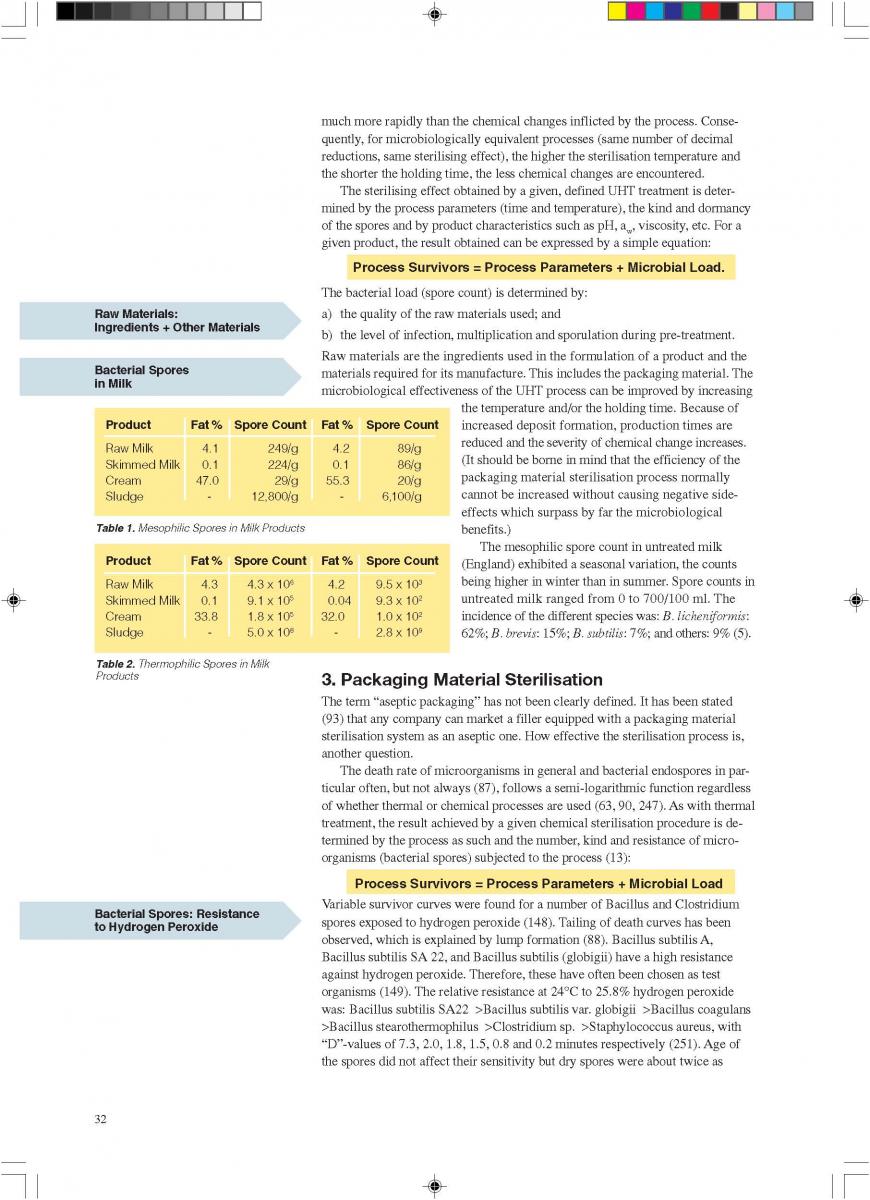
022. Sterilisation of the Packaging Material
2.Sterilisation of the Packaging Material
In aseptic packaging procedures, sterilisation of the packaging material (food contact surface) is achieved with few exceptions by chemical means (61). By far the most common chemical used for this purpose is hydrogen peroxide (H2O2) (93, 142). Of importance are the microbiological efficiency of the sterilisation process and the elimination of the chemical which might get into the filled product as a residue.
Depending on the make of the aseptic packaging equipment, different means of applying the sterilant are used: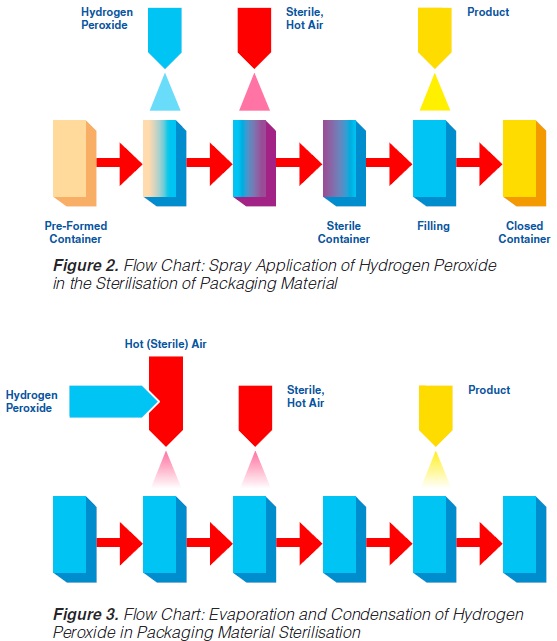
1) spray;
2) vapour;
3) roller systems;
4) immersion bath, etc.
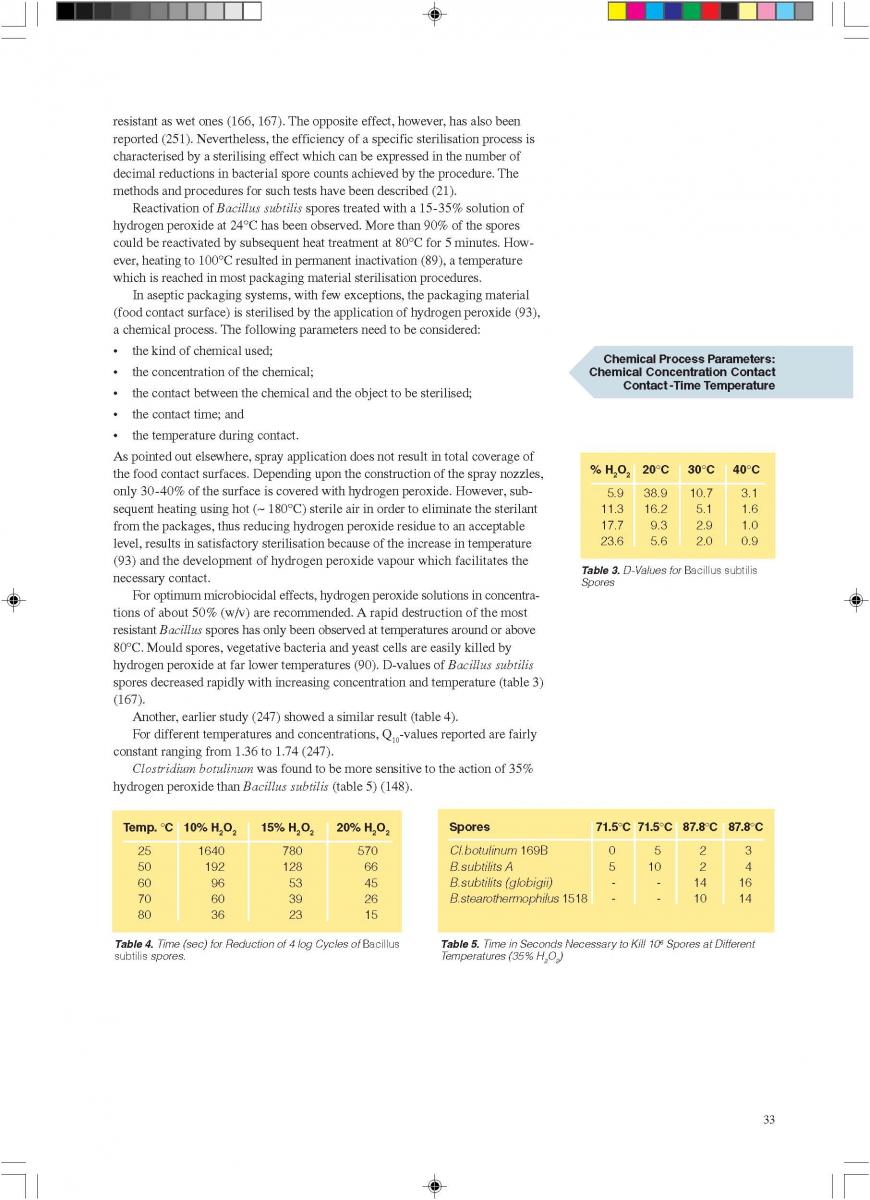
2.1 Spray Application
The spraying (“fogging”) of hydrogen peroxide is used in some aseptic packaging systems which operate intermittently and use pre-formed blanks (61, 74, 93, 171).
A certain amount of H2O2 is sprayed into each pre-erected container through a spray nozzle (figure 2). For proper function (sterilisation), the food contact surface of the container needs to be covered completely with the spray solution. Because of the hydrophobic characteristics of plastic materials in general and polyethylene in particular, it has been found (91, 145) that only 20-30% of the carton surfaces are wetted. In spite of this, good killing effects were achieved: up to 6 log reductions when tested with Bacillus subtilis spores have been reported (145), probably because of the subsequent evaporation.
Hot sterile air is blown into the container to attain the temperature necessary for the sterilisation process and to remove the H2O2 from the food contact surface. Using sterile air at
180°C for drying, 5 to 7 decimal reductions of Bacillus subtilis spores were attained (91, 113).
The killing efficiency of low concentrations (~ 0.5% or less) of hydrogen peroxide was greatly enhanced by the simultaneous use of ultraviolet radiation (44, 45).
2.2 Application by Vapour
In systems applying hydrogen peroxide by vaporisation, liquid H2O2 is injected into a stream of hot (sterile) air, then vaporised and condensed on the surfaces to be sterilised (93). A better coverage
of surfaces is obtained since the condensing droplets are smaller. Subsequently, the hydrogen peroxide is heated and evaporated by blowing hot, sterile air into the containers (figure 3).
2.3 Application by Roller System
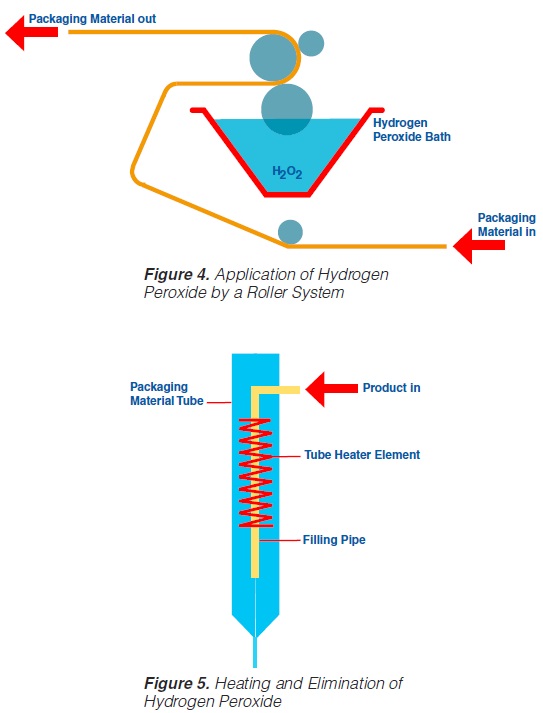
Roller systems (figure 4) permit the application of liquid hydrogen peroxide on to flat food contact surfaces (59, 60, 61, 74, 93). Packaging material sterilisation thus becomes possible before the containers are formed (figure 4).
In order to obtain a film of the water like H2O2 liquid covering the total food contact surface, a wetting agent – about 0.2 to 0.3% of PSM, (polyoxyethylenesorbitan-
monolaurate), or equivalent is recommended - has to be added to the hydrogen peroxide. After application of the sterilant on to the food contact surface, the packaging material web is formed into a tube and sealed longitudinally. The actual sterilisation requires the hydrogen peroxide covering the
packaging material food contact surface to be at a high temperature. An electrical element (“tube heater”) provides the temperature necessary (105-110°C) for the sterilisation process and, simultaneously, eliminates the H2O2 (figure 5).
2.4 Use of an Immersion Bath
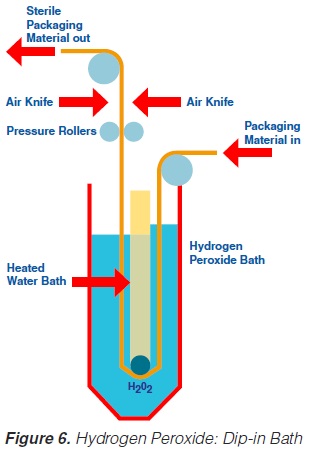
Likewise, the use of a hydrogen peroxide bath permits sterilisation of a plane packaging material web (59, 61, 69, 73, 74, 93, 171) prior to the actual forming of the container. The packaging material is sterilised by its passage through liquid hydrogen peroxide. The temperature necessary for the sterilisation process is obtained by heating the hydrogen peroxide solution indirectly by means of a water bath which is placed inside the H2O2 bath (figure 6). A concentration of 30%, a temperature of 70°C and an exposure time of about 10 seconds are necessary to achieve sufficient killing of bacterial endospores (74). In some models, the hydrogen peroxide is removed from the packaging material:
a) by a pair of pressure rollers that squeeze the excess chemical back into the hydrogen peroxide bath; and
b) by a pair of air knives that blow hot, sterile air on to both sides of the packaging material web.
After passage through the bath and removal of the hydrogen peroxide, the sterile, flat packaging material web is formed into a tube and sealed longitudinally.
04. H2O2 as a sterilant


05. Packaging material


06. Application of microbiology to UHT processing and aseptic packaging

07. Installation


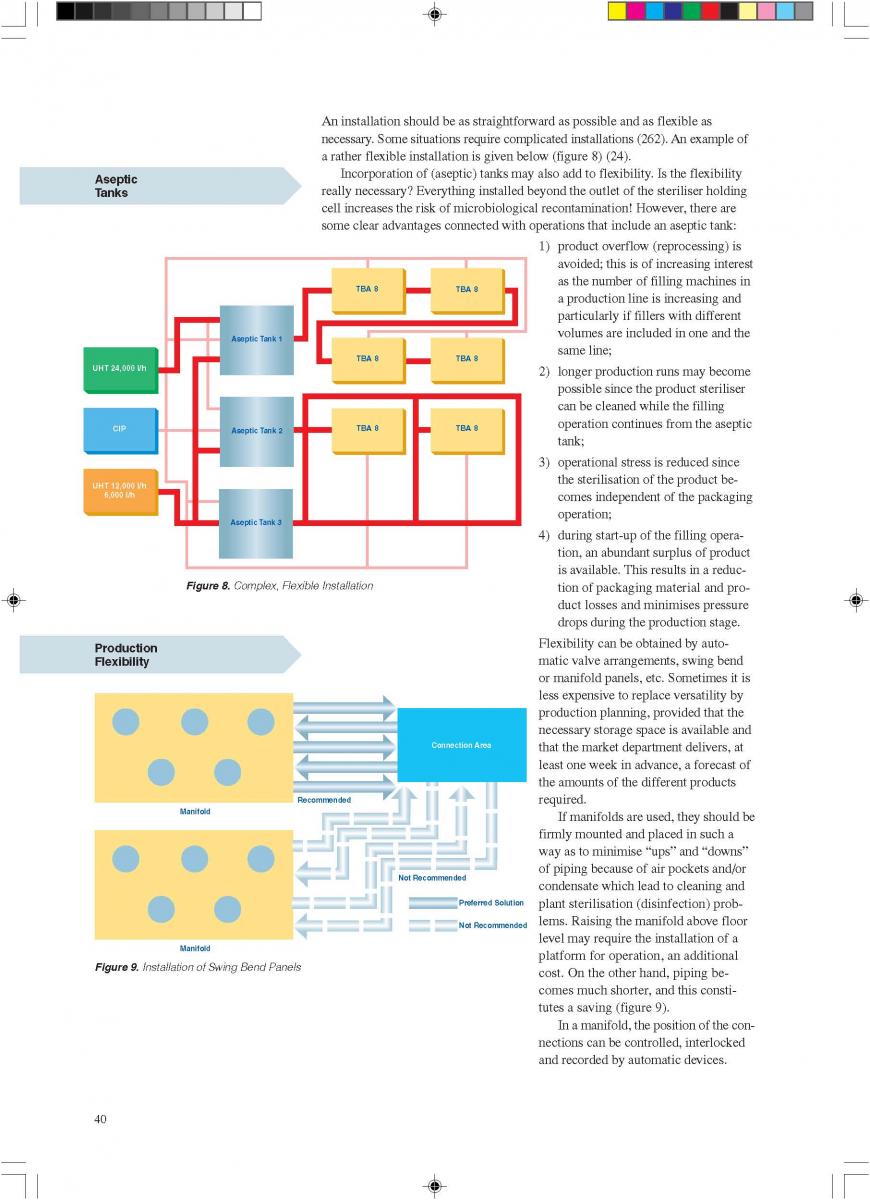
08. Cleaning and house keeping



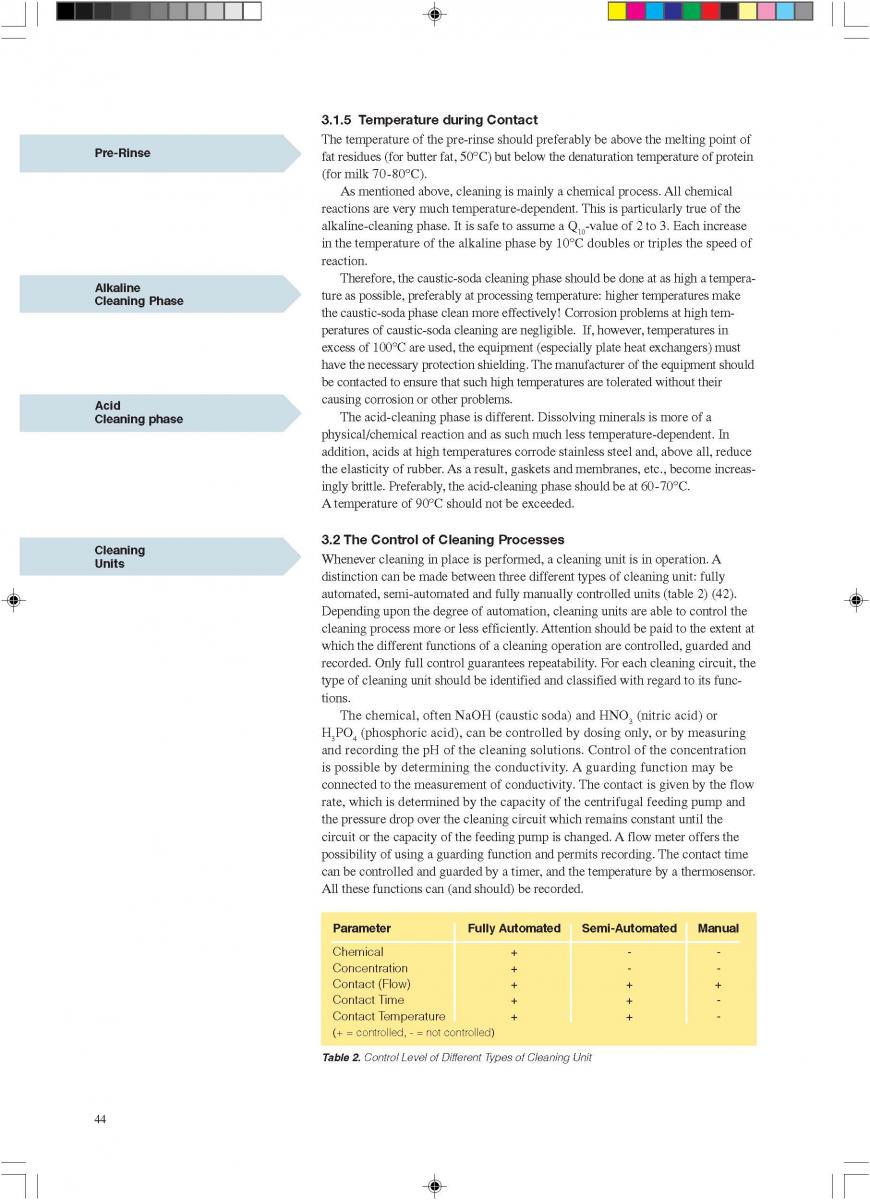
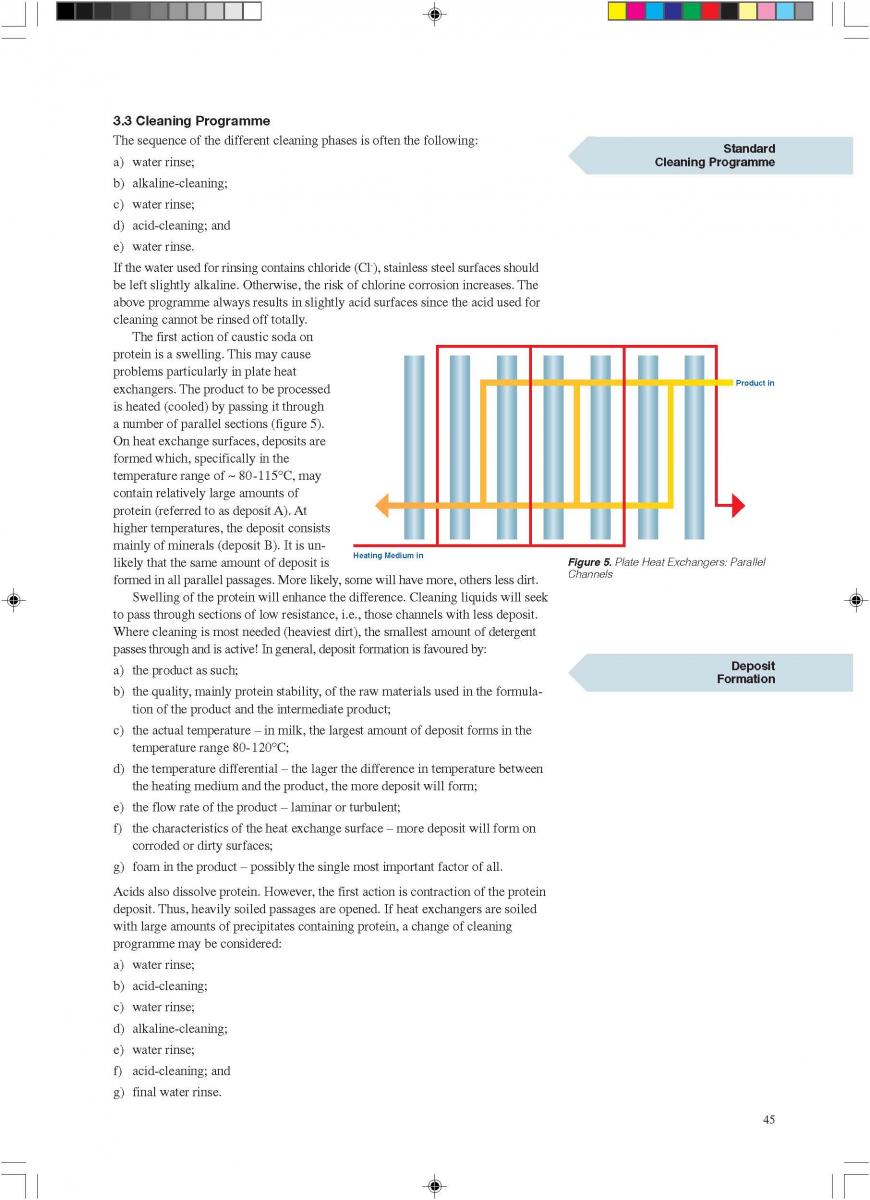


09. Commissioning
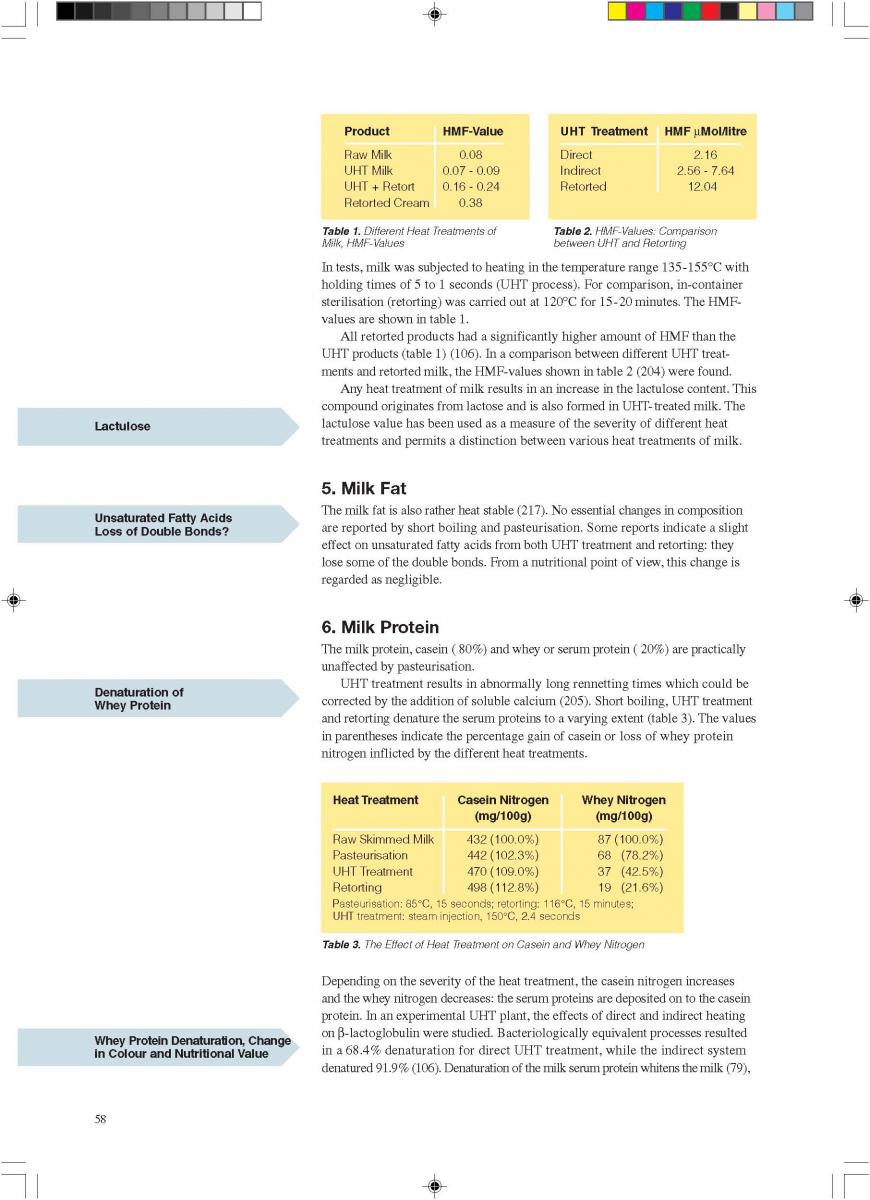
10. Changes during milk processing

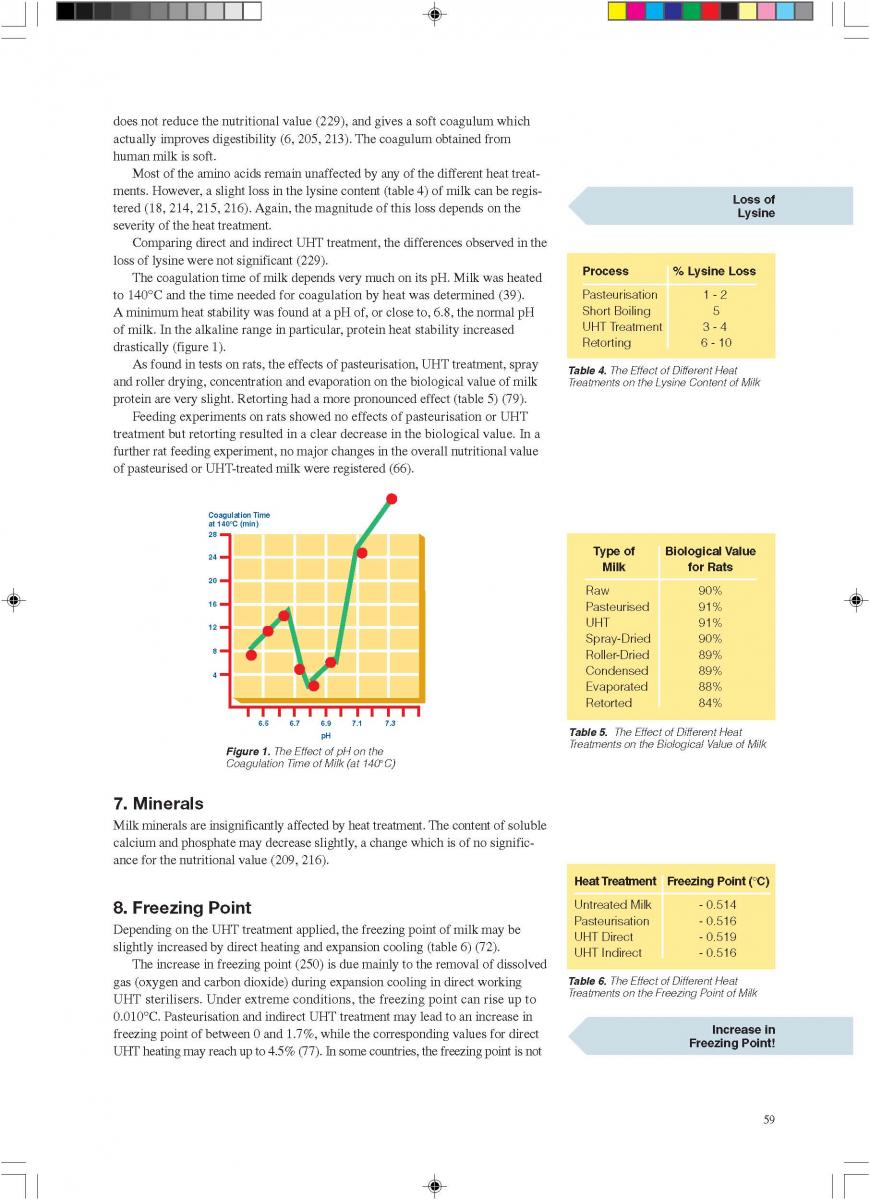

11. Product changes during storage


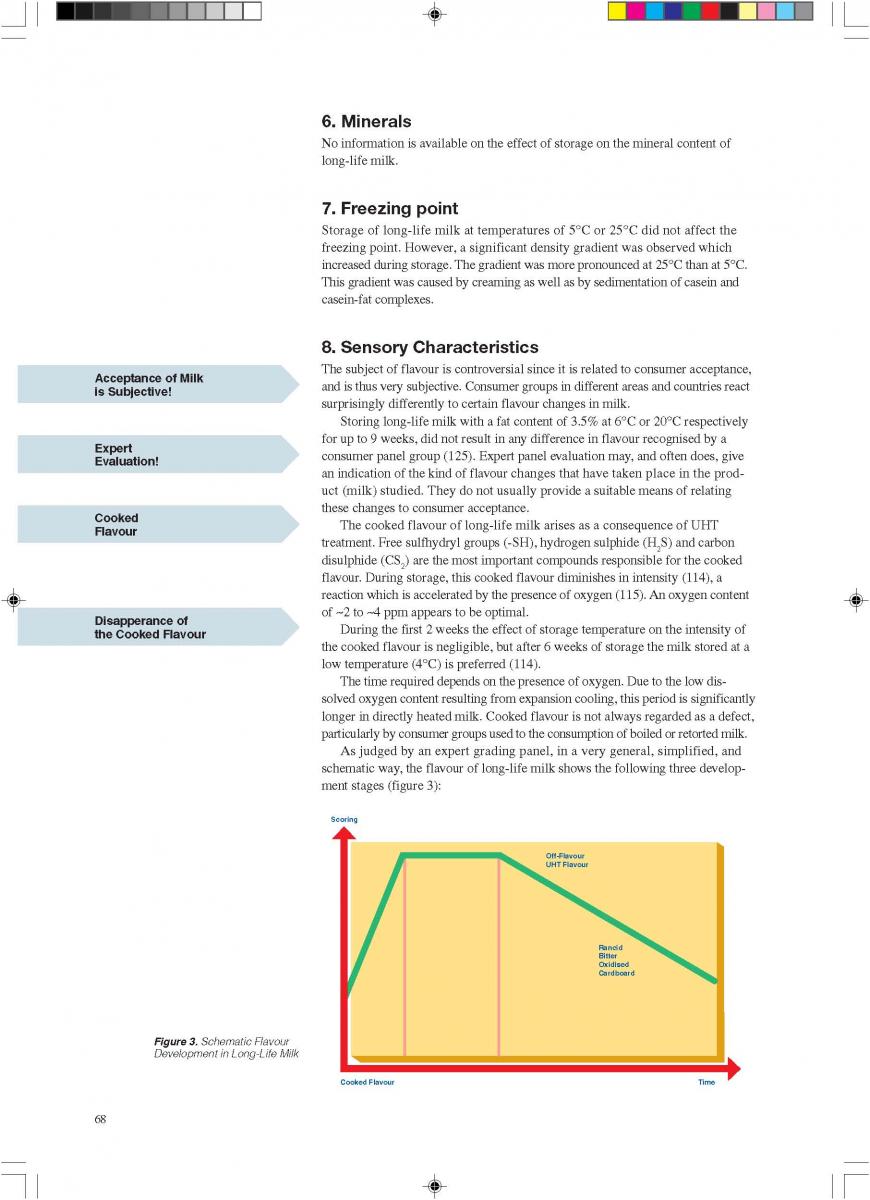

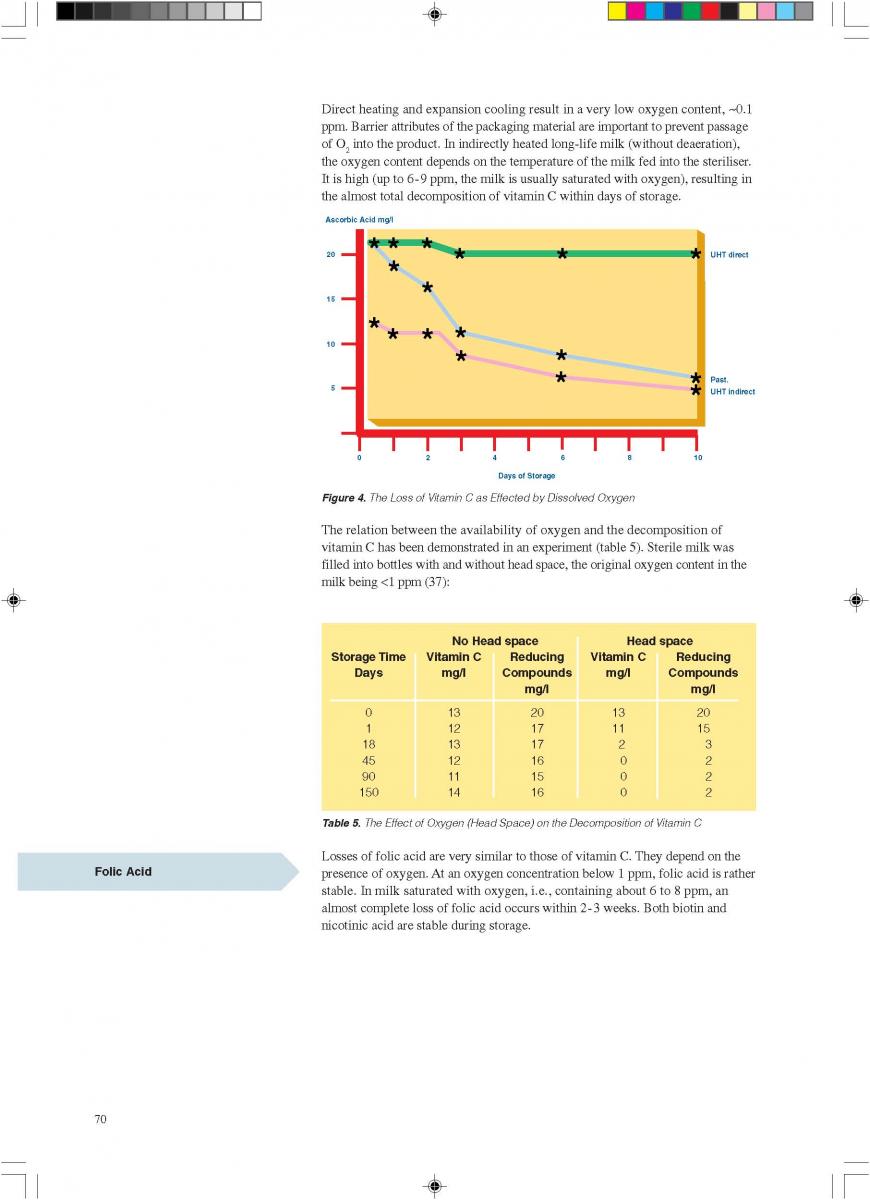
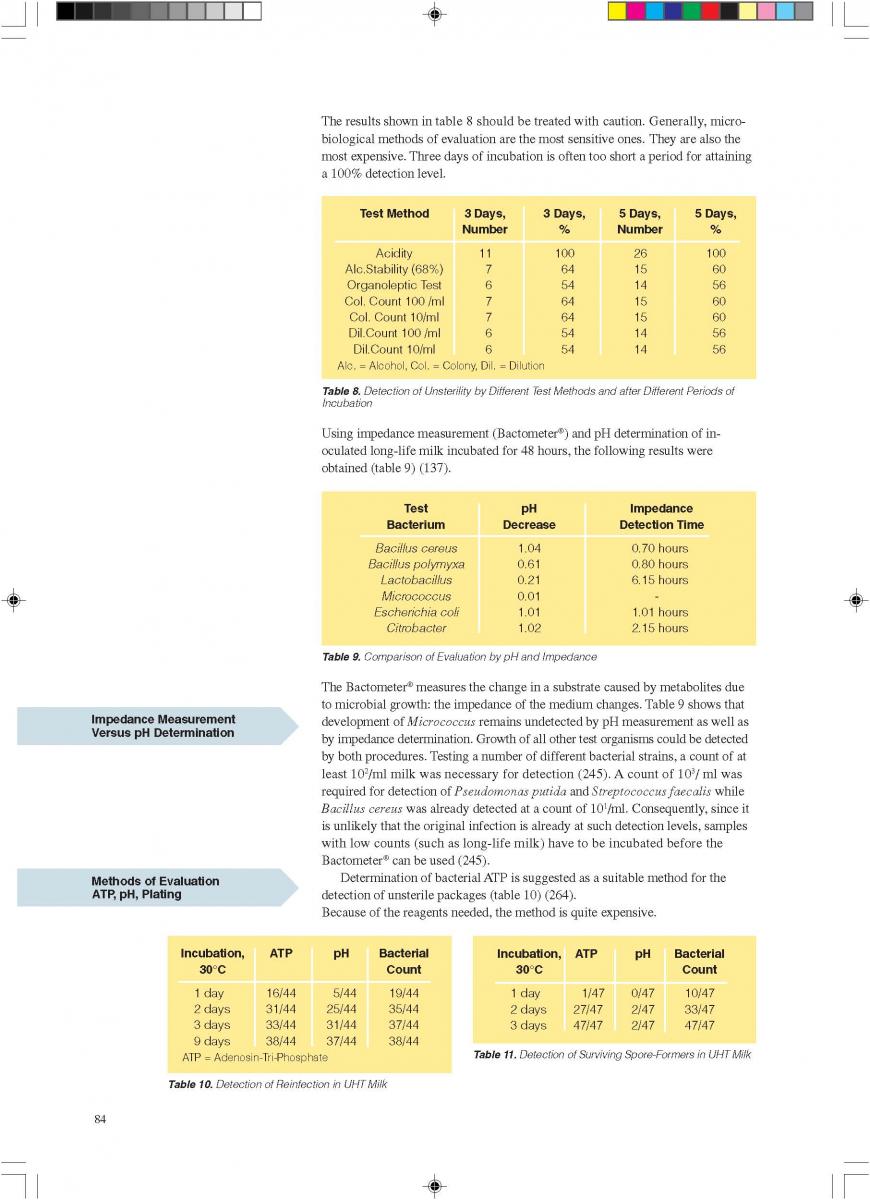
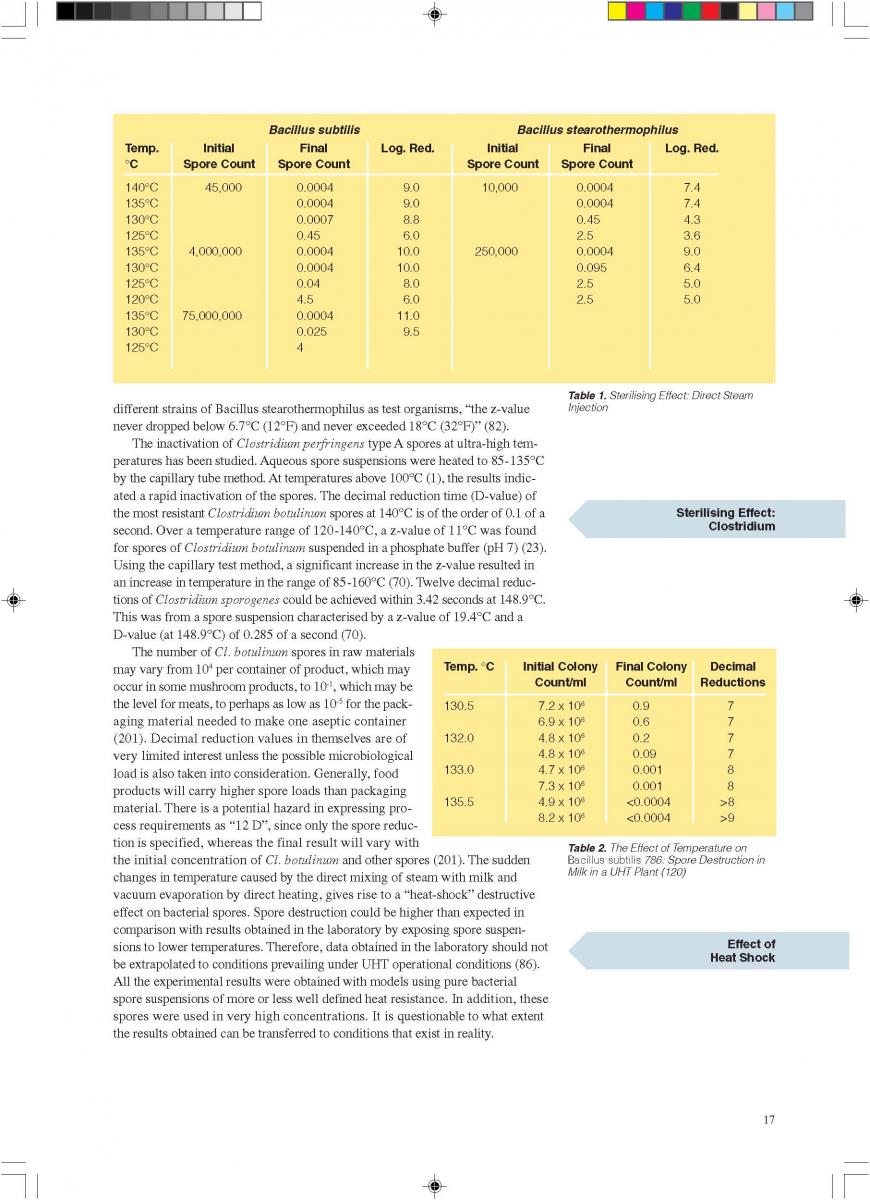
12. Quality control of aseptic product-Part1
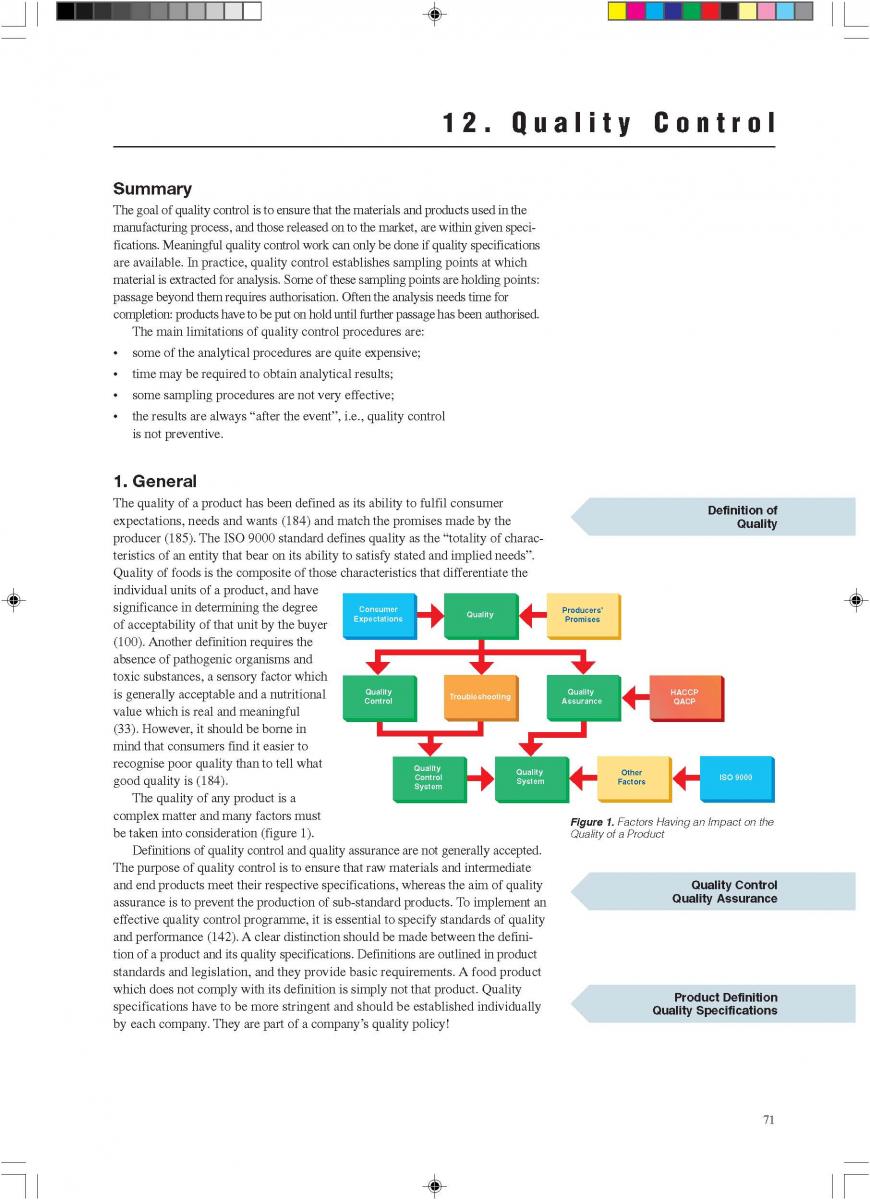


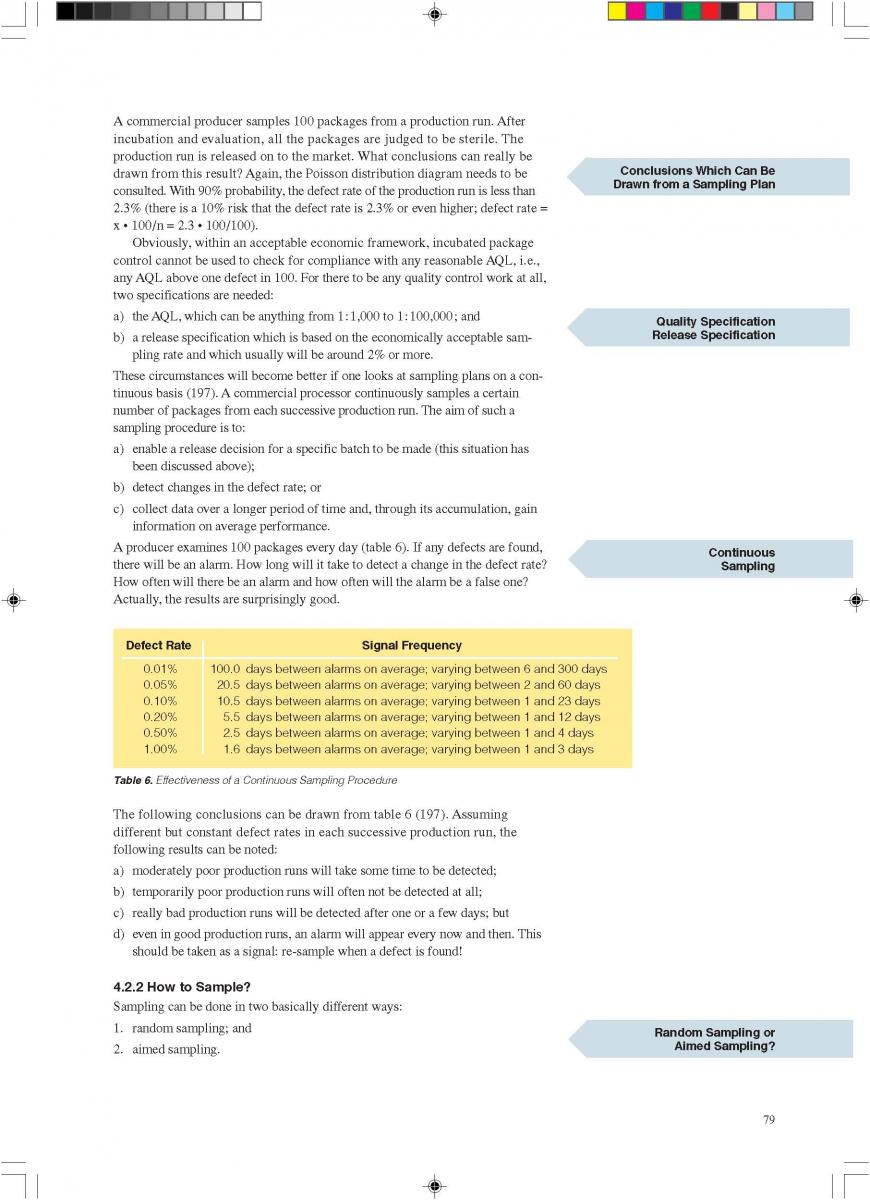
12. Quality control of aseptic product-Part2